-40%
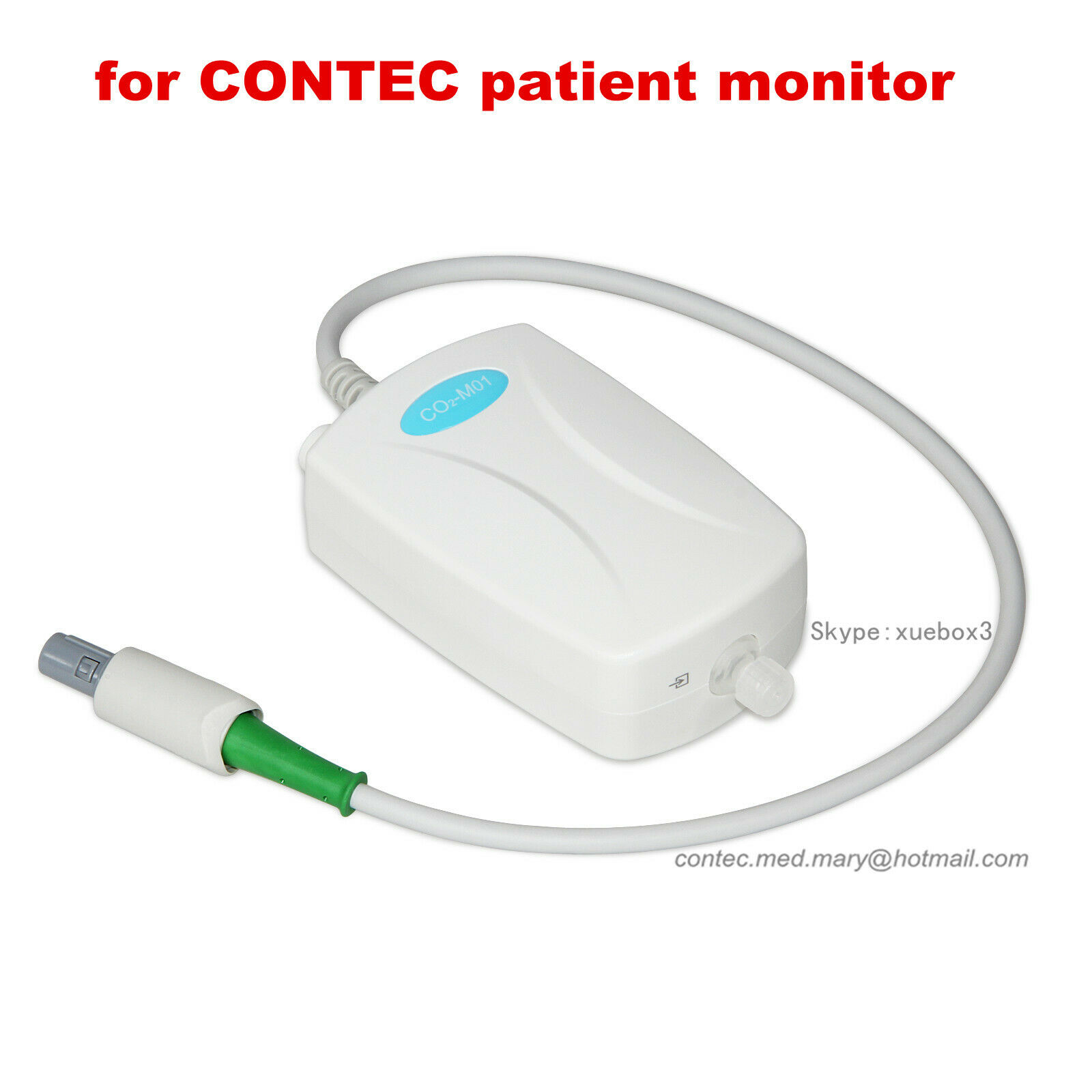
Capnography EtCO2 Respiratory Gas CO2 Monitor Module,Sidestream for patient NEW
$ 157.87
- Description
- Size Guide
Description
When you buy it,please let me know your machine model at first.so that you can receive the compatible one,ThanksIntroduction
Introduction
CO2-M01 Respiratory Gas CO2 Monitor Module is a clinical equipment for continuous monitoring CO2. Common Infrared absorption spectroscopy is divided into mainstream and sidestream according to gas sampling mode, and this module is designed for sidestream measurement. The module provides patient's physiological state for physician or nurse in time, which plays an important role in clinical anesthesia, CPCR(Cardiac Pulmonary Cerebral Resuscitation), ICU(intensive care unit) and emergency medicine, etc. It can be used under physician monitoring, and it is be applicable for adult, pediatric and neonatal.
Features
Continuously measure CO2 concentration in gas, and calculate End-tidal CO2(EtCO2),Inspired Minimum CO2(InsCO2 ), AirWay Respiration Rate(AwRR).
Elegant in appearance, and clear in labelling.
Simple operation, safe and effective, which meets the requirements of relative standards.
Three units (mmHg, kPa and %) are optional.
Performance
CO2 Measurement Range: 0 to 150 mmHg
CO2 Measurement Accuracy: 0~40 mmHg: ±2mmHg
41~70 mmHg: ±5% of reading
71~100 mmHg: ±8% of reading
101~150 mmHg: ±10% of reading
Respiratory monitoring range: 2rpm~150rpm, accuracy: ±1rpm
Working voltage: DC 5.0V
Physical characteristic
Dimension:103mm(L)×69mm(W)×39mm(H)
Net weight: 141g
Buy safe products
The following FDA Disclaimer is required for all eBay listing in Healthcare category and is included for REFERENCE: The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If the item is subject to FDA regulation, we will verify your status as an authorized purchaser of this item before shipping of the item.
The Fingertip Pulse Oximeter is registered on the Australian Register of Therapeutic Goods (ARTG)with the code 197923, and certified by FDA of United States and CE,TUV of Europe. The Fingertip Pulse Oximeter that is FDA 510K Approved
International Buyer--Please note:
Import duties,Taxes are not included in the item price.These charges are buyers' responsibility
We are the manufacturer of medical equipment for 17 years, the following are our main products:
ECG / ECG holter EEG / EEG Holter B-ultrasound
Patient Monitor Fetal doppler Fetal Monitor
Spo2 Monitor Stethoscope Medical Image...
.
Agent in the world!
We are look for partners in the world now!
Do you want to become our agent in you conutry?
Join US!
Skype:xuebox3
contec.med.mary # hotmail dot com
Only English User Manual,Please contact us if you need other language version.thank you