-40%
CONTEC ICU CCU Patient Monitor 6 Parameters CO2 Vital Signs Monitor CMS6000C CE
$ 189.55
- Description
- Size Guide
Description
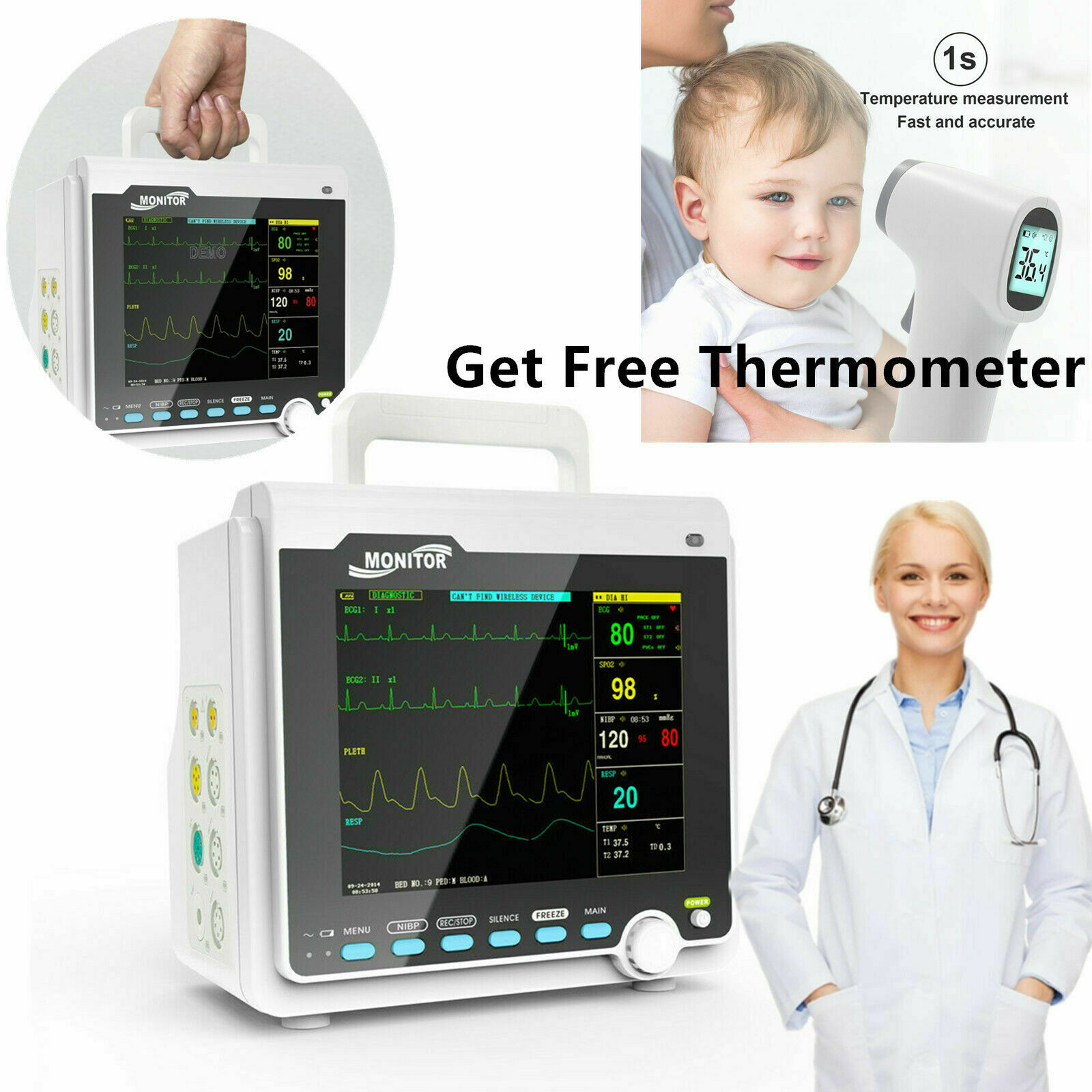
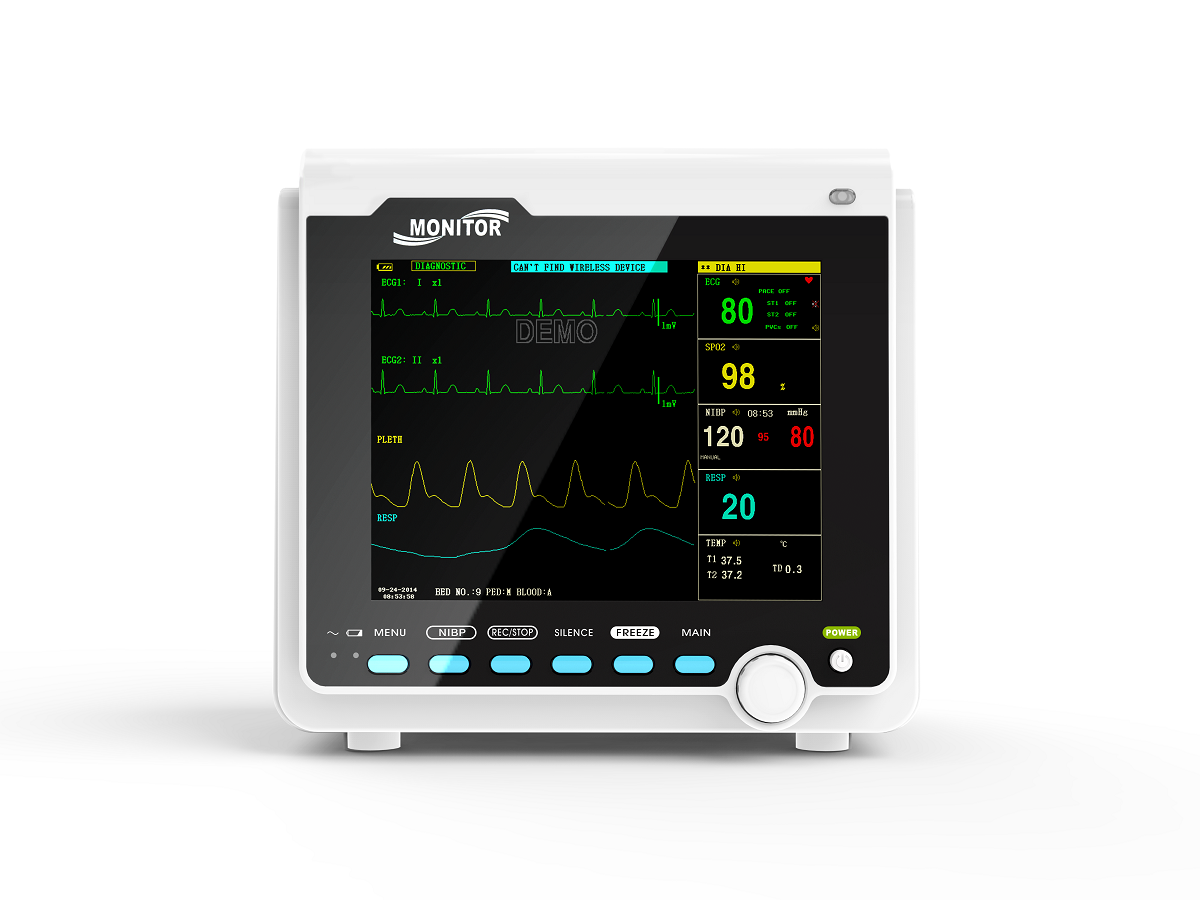
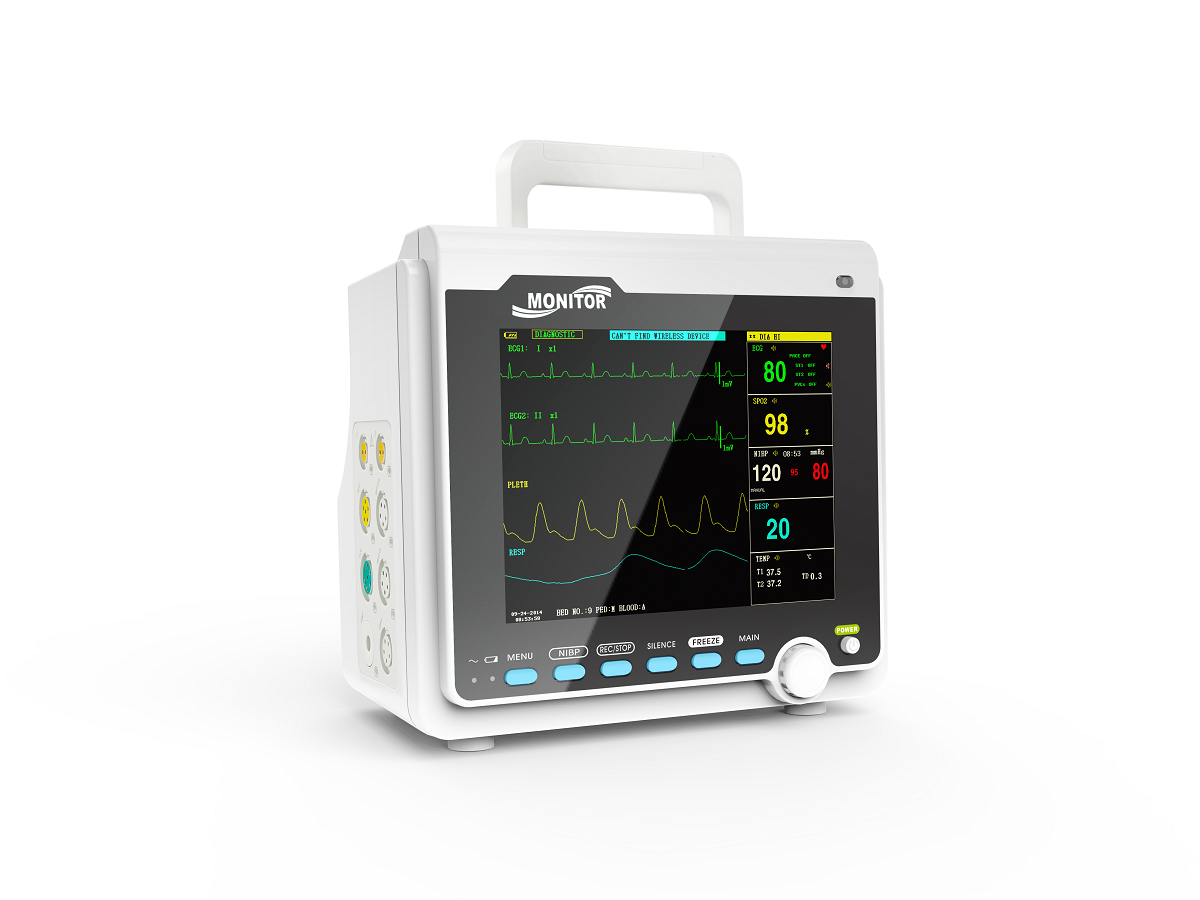
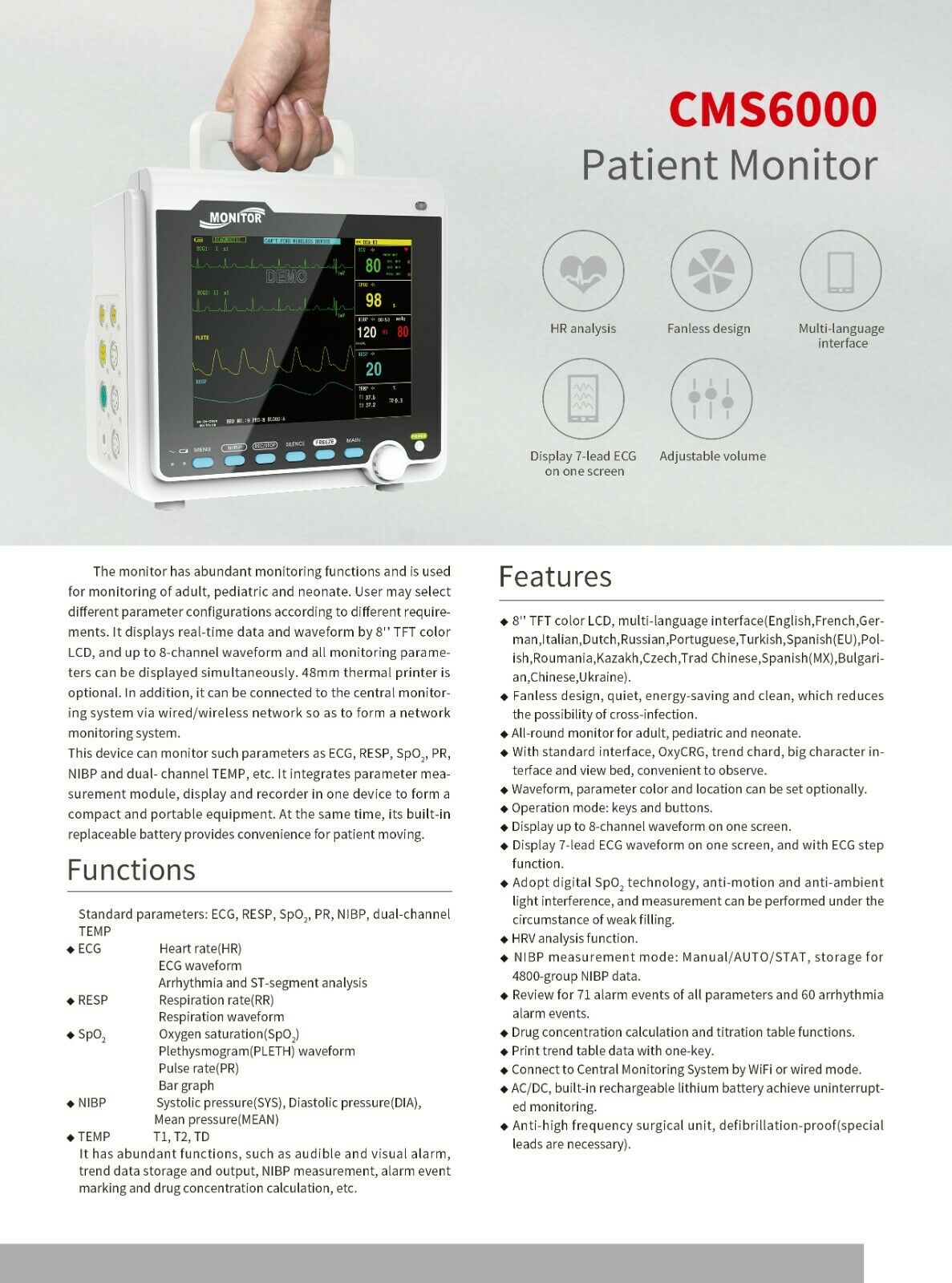
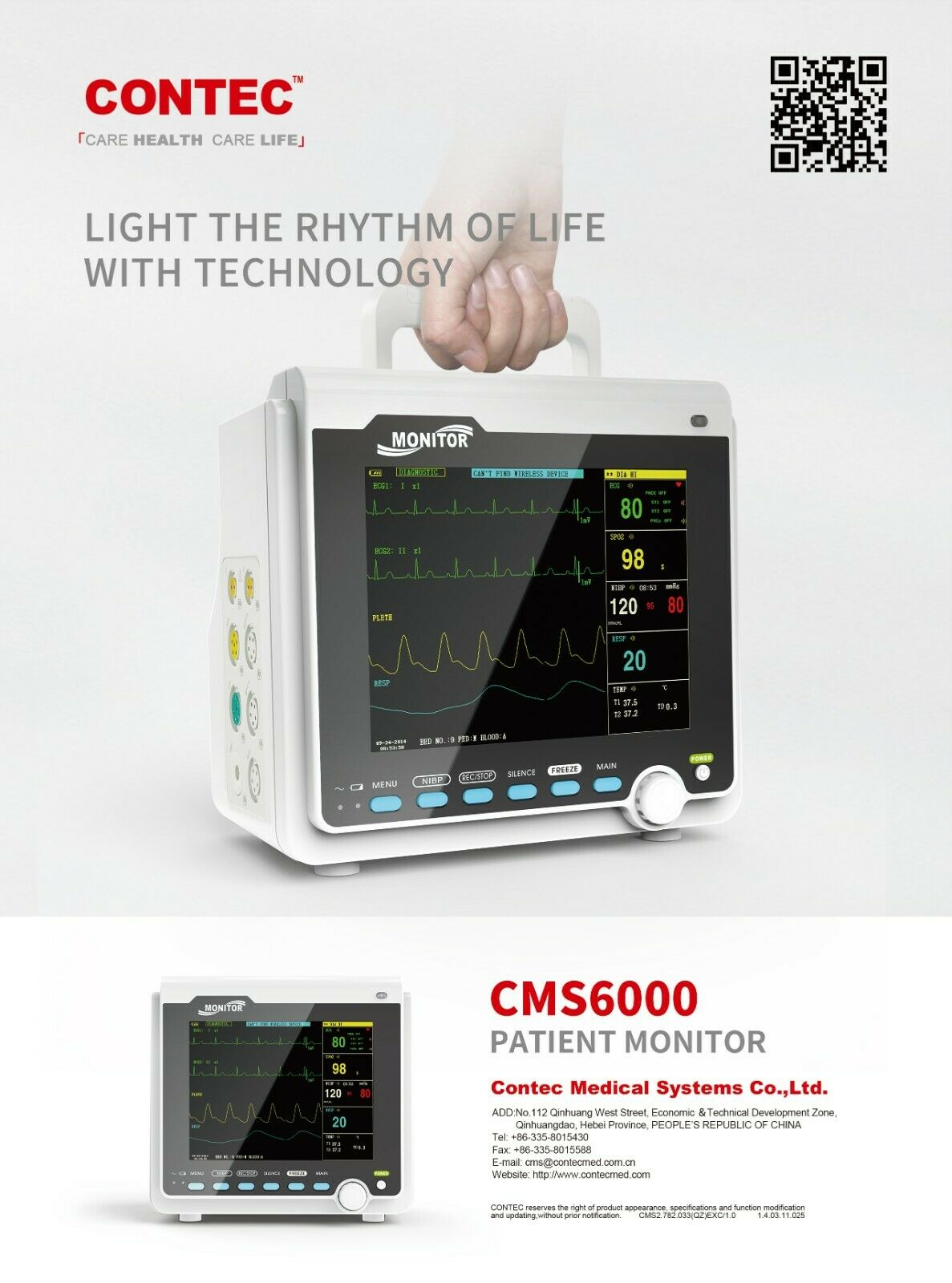
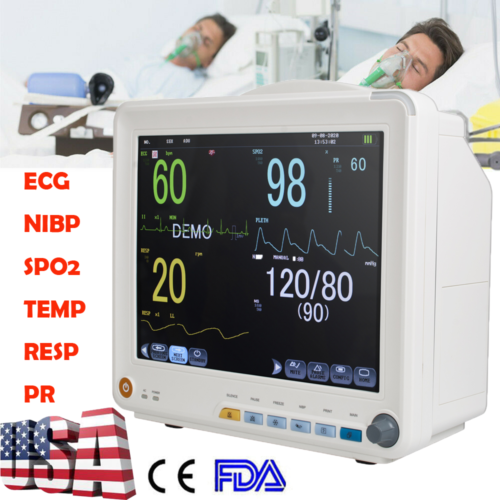
IntroductionThe monitor has abundant monitoring functions and is used for monitoring of adult, pediatric and neonate. User may select different parameter configurations according to different requirements. It displays real-time data and waveform by 8'' TFT color LCD, and up to 8-channel waveform and all monitoring parameters can be displayed simultaneously. 48mm thermal printer is optional. In addition, it can be connected to the central monitoring system via wired/wireless network so as to form a network monitoring system.
This device can monitor such parameters as ECG, RESP, SpO2, PR, NIBP and dual- channel TEMP, etc. It integrates parameter measurement module, display and recorder in one device to form a compact and portable equipment. At the same time, its built-in replaceable battery provides convenience for patient moving.
Functions
Standard parameters: ECG, RESP, SpO2, PR, NIBP, dual-channel TEMP
1)ECG Heart rate(HR)
ECG waveform
Arrhythmia and ST-segment analysis
2)RESP Respiration rate(RR)
Respiration waveform
3)SpO2 Oxygen saturation(SpO2)
Plethysmogram(PLETH) waveform
Pulse rate(PR)
Bar graph
4)NIBP Systolic pressure(SYS), Diastolic pressure(DIA), Mean pressure(MEAN)
5)TEMP T1, T2, TD
It has abundant functions, such as audible and visual alarm, trend data storage and output, NIBP measurement, alarm event marking and drug concentration calculation, etc.
Features
1)8'' TFT color LCD, multi-language interface.
2)Fanless design, quiet, energy-saving and clean, which reduces the possibility of cross-infection.
3)All-round monitor for adult, pediatric and neonate.
4)With standard interface, OxyCRG, trend chard, big character interface and view bed, convenient to observe.
5)Waveform, parameter color and location can be set optionally.
6)Operation mode: keys and buttons.
7)Display up to 8-channel waveform on one screen.
8)Display 7-lead ECG waveform on one screen, and with ECG step function.
9)Adopt digital SpO2 technology, anti-motion and anti-ambient light interference, and measurement can be performed under the circumstance of weak filling.
10)HRV analysis function.
11)NIBP measurement mode: Manual/AUTO/STAT, storage for 4800-group NIBP data.
12)Review for 71 alarm events of all parameters and 60 arrhythmia alarm events.
13)Drug concentration calculation and titration table functions.
14)Print trend table data with one-key.
15)Connect to Central Monitoring System by 3G, WiFi or wired mode.
16)AC/DC, built-in rechargeable lithium battery achieve uninterrupted monitoring.
17)Anti-high frequency surgical unit, defibrillation-proof(special leads are necessary).
Performance
▲ECG
Lead mode: 3-lead or 5-lead
Lead selection: I, II, III, aVR, aVL, aVF, V
Waveform: 5-lead, 2-channel
3-lead, 1-channel
Gain: 2.5mm/mV, 5.0mm/mV, 10mm/mV, 20mm/mV, 40mm/mV
Scan speed: 12.5mm/s, 25 mm/s, 50 mm/s
HR:
Measurement and alarm range: 15~350bpm
Accuracy: ±1% or ±1bpm, whichever is greater
Alarm accuracy: ± 2 bpm
Resolution: 1 bpm
ST-segment monitoring:
Measurement and alarm range: -2.0mV~+2.0mV
Accuracy: -0.8mV~+0.8mV ±0.04mv or ±10%, whichever is greater
Other range: unspecified
Arrhythmia analysis: ASYSTOLE, VFIB/VTAC, COUPLET, BIGEMINY, TRIGEMINY, R ON T, VT>2, PVC, TACHY, BRADY, MISSED BEATS, PNP, PNC
Pacemaker: yes
RESPIRATION
Method: R-F(RA-LL) Impedance
Respiration rate:
Measurement and alarm range: 0~150rpm
Resolution: 1 rpm
Measurement accuracy: ±2 rpm
Alarm accuracy: ±3 rpm
Apnea alarm: 10~40s
Scan speed: 6.25 mm/s, 12.5 mm/s, 25 mm/s
▲NIBP
Method: Oscillometry
Mode: Manual/AUTO/STAT
Measurement interval in AUTO mode: 1/2/3/4/5/10/15/30/60/90/120/240/480/960 minutes
Measurement period in STAT mode: 5 minutes
Measurement and alarm range: 10 ~ 270 mmHg
Resolution: 1 mmHg
Cuff pressure accuracy: ±3 mmHg
Measurement accuracy:
Maximal mean deviation: ±5 mmHg
Maximal standard deviation: 8mmHg
Over-pressure protection:
Adult mode: 297±3 mmHg
Pediatric mode: 240±3 mmHg
Neonatal mode: 147±3 mmHg
▲SpO2
Measurement and alarm range: 0 ~ 100%
Resolution: 1%
Measurement accuracy: 70%~100%: ±2%;
0%~69%: unspecified
PR
Measurement and alarm range: 30 ~ 250 bpm
Measurement accuracy: ±2 bpm or ±2%, whichever is greater
TEMP
Channel: dual-channel
Measurement and alarm range: 0 ~ 50℃
Resolution: 0.1 ℃
Accuracy: ±0.1 ℃
Accessories
Adult fingertip SpO2 probe (5-pin)
Adult NIBP cuff
NIBP extension tube
ECG lead cable
ECG electrode
Temperature probe
Power adapter
Power cord
User Manual
Physical characteristic
Dimension: 235 mm(L) × 141 mm(W) × 222 mm(H)
Weight: 2.5 Kg
Payment
We accept Paypal Payment.
Shipping
Clearance: we will ship your item to your confirmed address.
Buyers' responsibility to pay duties,taxes and other extra charges by the government in your country.
Terms of Sale
Buy safe Products
The following FDA Disclaimer is required for all eBay listing in Healthcare category and is included for REFERENCE: The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies.If the item is subject to FDA regulation, We will verify your status as an authorized purchaser of this item before shipping of the item. If you have questions about legal obligations regarding sales of medical devices, you should consult with the FDA's Center for Devices and Radiological Health.
The Fingertip Pulse Oximeter is registered on the Australian Register of Therapeutic Goods (ARTG) with the code 197923, and certified by FDA of United States and CE,TUV of Europe.The Fingertip Pulse Oximeter that is FDA 510K Approved.
About Us
Contec Medical Systems Co.,Ltd; 20 Years manufacturer,we have stock in USA and China.