-40%
Portable Vital Sings Monitor ICU Patient Monitor NIBP SPO2 PR CMS5100 Color New
$ 157.87
- Description
- Size Guide
Description
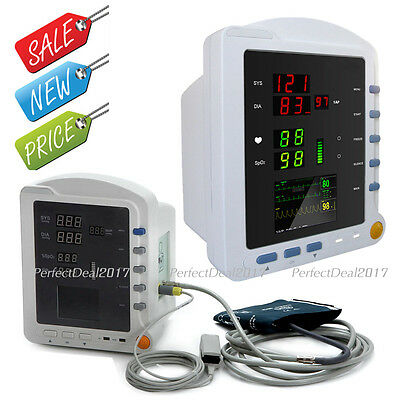
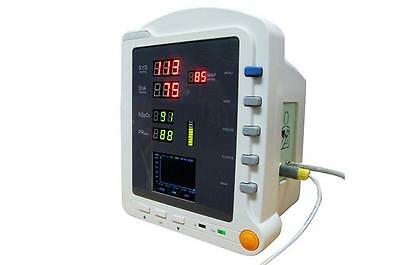
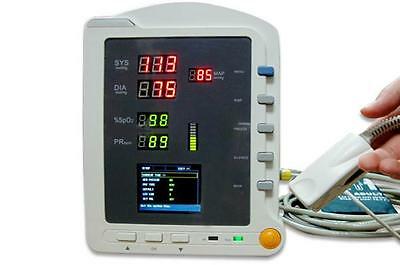
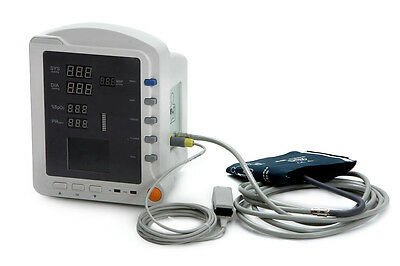
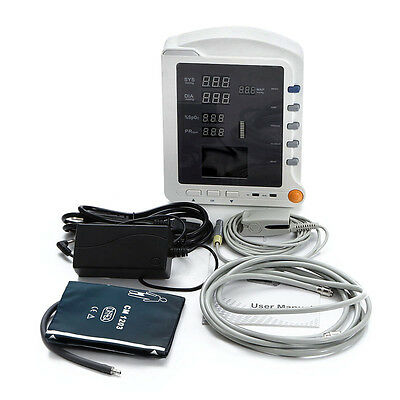
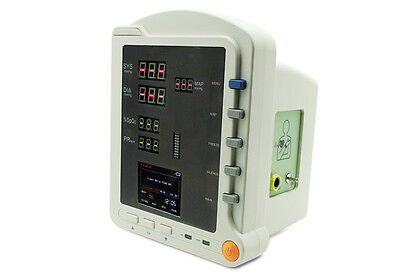
CMS5100 ICU Patient MonitorDescription
Model:CMS5100 Patient Monitor
Introduction
CMS5100 Patient Monitor is a feature-rich monitoring device that can be applied to adults, pediatric and neonate. User can choose different measurement parameters according to different needs. The patient monitor can monitor NIBP, SpO2 and PR. It integrates the function of parameter measuring and displaying into a compact, lightweight patient monitor, which is suitable for all levels of hospitals, community medical and home use.
Features
1)Be applicable for NIBP and SpO2 monitoring of adult, pediatric and neonate of all ages, easy to operate, high cost-effective;
2)Be applicable for medicine, surgery, operating room, ICU/CCU, emergency room, obstetrics and gynecology, pediatrics;
3)Compact and flexible appearance, easy for carrying and suitable for indoor and outdoor (in ambulance) monitoring;
4)Built-in rechargeable lithium polymer battery, ensuring uninterrupted monitoring;
5)Perfect menu design, user-friendly interface;
6)Display the measurement result of SYS, DIA, MAP, SpO2, PR and bar graph by the display screen of high-brightness digital tube;
7)2.8'' (320×240) true color TFT LCD screen, displays the information of time, SpO2 Plethysmogram, alarm condition, trend graph, list and system settings, etc.;
8)Visual and audible alarm for SYS, DIA, MAP, SpO2 and PR, and upper and lower limit of alarm can be set as necessary;
9)Independent nonvolatile memory, storage for up to 2,000 groups of NIBP data and 78,000 groups of SpO2 data;
10)Convenient and quick in reviewing measurement data, available for reviewing the NIBP trend graph of 24 hours and SpO2 trend graph of 22 hours.
Performance
1 NIBP
Using Oscillometry technology, also called Oscillography technology to measure the blood pressure.
1)Measurement mode: manual/auto/continuous
2)Measurement interval in auto mode: 1, 2, 3, 4, 5, 5×n(n=2, 3, 4...51) min.
3)Resolution: 1 mmHg
4)Accuracy: max mean error: ±5 mmHg; max standard deviation: 8 mmHg.
5)Overpressure protection: dual protection for both software and hardware
6)Others: reset, self-testing and accuracy testing of static pressure
7)Measurement range:
Adult
SYS 40 mmHg~270 mmHg
MAP 20 mmHg~235 mmHg
DIA 10 mmHg~215 mmHg
Pediatric:
SYS 40 mmHg~200 mmHg
MAP 20 mmHg~165 mmHg
DIA 10 mmHg~150 mmHg
Neonate:
SYS 40 mmHg~135 mmHg
MAP 20 mmHg~110 mmHg
DIA 10 mmHg~100 mmHg
2 SpO2
Using Photoelectric Oxyhemoglobin Inspection Technology combined with Capacity Pulse Scanning & Recording Technology to measure the saturation of blood oxygen.
1)Measurement range: 0 %~100 %
2)Alarm range: 0 %~100 %
3)Resolution: 1 %
4)Accuracy: 70 %~100 %, ±2 %; below 70 % unspecified.
3 PR
Using Photoelectric Oxyhemoglobin Inspection Technology combined with Capacity Pulse Scanning & Recording Technology to measure the pulse rate.
1)Measurement range: 25 bpm~250 bpm
2)Alarm range: 25 bpm~250 bpm
3)Resolution: 1 bpm
4)Accuracy: ±2 bpm or ±2 %, whichever is greater
4 Safety and Power supply
1)Power supply: rated voltage: ~220 V, rated power: 50 Hz
2)Safety classification: Class I equipment, type BF defibrillation-proof applied part
Accessories
1)User Manual 1pc
2)Power cord 1pc
3)Power adapter 1pc
4)SpO2 sensor 1pc
5)Adult NIBP cuff 1pc
6)NIBP extension tube 1pc
Physical characteristic
Dimension: 190 mm(L)×162 mm(W)×240 mm(H)
Weight: about 1.6 kg
1.Paypal Account
2.Bank Transfer
Shipping
Clearance: we will ship your item to your Ebay confirmed address.
Buyers' responsibility to pay duties,taxes and other extra charges by the government in your country.
All items will be shipped within 2 business days by E-package,Airmail shipping.
According to shipping method,items will be delivered within 7-35 days.
We will do low value in PI,so that you can get items from your customs smoothly.
Terms of Sale
Buy safe Products
The following FDA Disclaimer is required for all eBay listing in Healthcare category and is included for REFERENCE:
The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies.If the item is subject to FDA regulation, We will verify your status as an authorized purchaser of this item before shipping of the item.
If you have questions about legal obligations regarding sales of medical devices, you should consult with the FDA's Center for Devices and Radiological Health.
The Fingertip Pulse Oximeter is registered on the Australian Register of Therapeutic Goods (ARTG) with the code 197923, and certified by FDA of United States and CE,TUV of Europe.The Fingertip Pulse Oximeter that is FDA 510K Approved
About Us
Contec Medical Systems Co.,Ltd; 20 Years medical equipment manufacturer,we have stock in USA and China.
We supply 60 days return policy and 3 years warranty.
Contact Us
Contact Name:Tessy Zhang;
Powered by SoldEazy
Only English user guide, if you need any other language, please contact us!